In our daily lives, batteries are omnipresent, powering everything from remote controls to electric vehicles. But have you ever wondered what sets regular batteries apart from lithium batteries? Which one is more durable? Today, let’s delve into the world of batteries and uncover their mysteries!
I. Chemical Composition: Different “Recipes,” Different Performances
Regular Batteries
Regular batteries come in various types, with zinc-manganese dry batteries being the most common. The negative electrode is zinc, while the positive electrode is manganese dioxide. When the battery is in use, zinc undergoes oxidation by losing electrons, and manganese dioxide gains electrons through reduction.
The electrolyte in these batteries is typically a mixture of ammonium chloride and zinc chloride, which together facilitate the electrochemical reactions that generate electricity. This simple chemical combination makes zinc-manganese dry batteries cost-effective but with lower energy density, making them suitable for small electronic devices with low power requirements, such as remote controls and small radios.

Lithium Batteries
The chemical makeup of lithium batteries is more complex. The negative electrode is primarily composed of lithium metal or lithium alloy, and the positive electrode can be one of several materials, including lithium cobalt oxide (LiCoO₂), lithium manganese oxide (LiMn₂O₄), and lithium iron phosphate (LiFePO₄). Taking lithium cobalt oxide as an example, during charging, lithium ions are released from the positive electrode material, travel through the electrolyte, and are embedded into the negative electrode material.
When discharging, the lithium ions move back from the negative electrode to the positive electrode. The electrolyte is usually a mixture of organic solvents and lithium salts, such as carbonate solvents and lithium hexafluorophosphate (LiPF₆). This electrolyte system allows lithium ions to shuttle quickly between the positive and negative electrodes, enabling the battery’s charge and discharge processes. The high energy density and long cycle life of lithium batteries make them the ideal choice for modern electronic devices.

II. Performance Features: Who is the True “Energy King”?
Energy Density
-
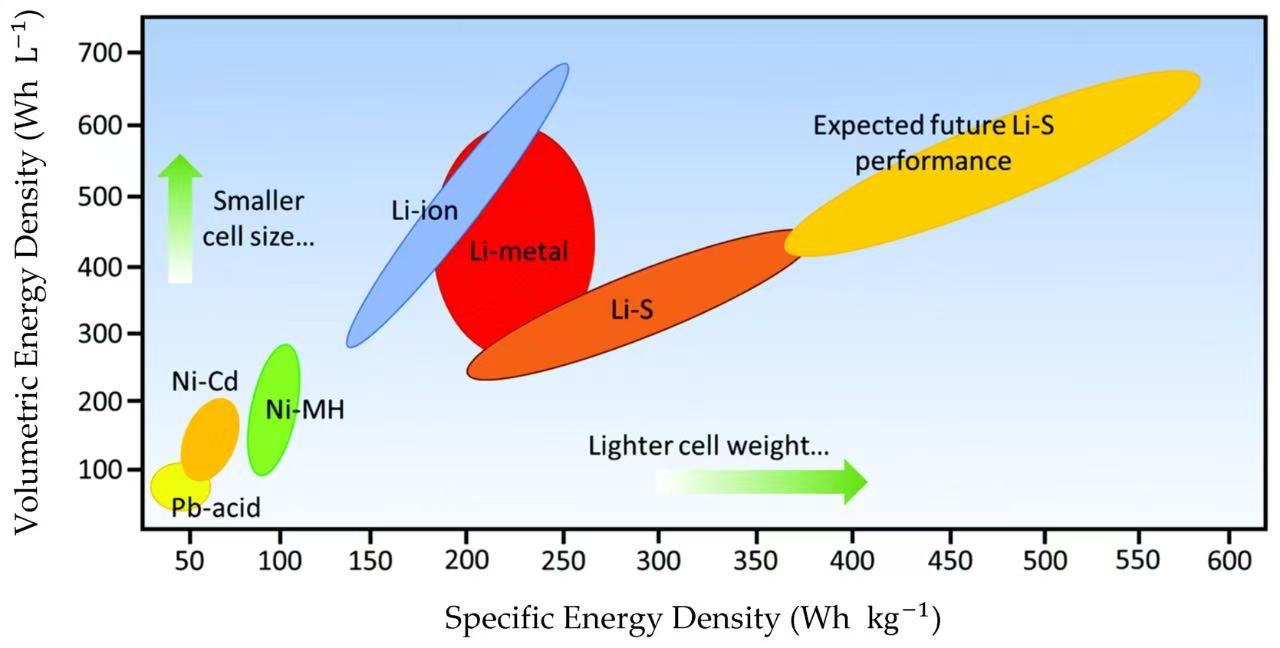
Regular Batteries: The energy density of zinc-manganese dry batteries is relatively low, around 100 - 130Wh/kg. This means that for a given weight, the amount of energy they can provide is limited, making them suitable for small electronic devices with low power demands.
-
Lithium Batteries: Lithium batteries have a much higher energy density. For example, the energy density of lithium iron phosphate batteries is generally between 120 - 180Wh/kg, while high-performance ternary lithium batteries (such as nickel cobalt manganese oxide) can reach 200 - 300Wh/kg. The high energy density allows lithium batteries to store more electrical energy in the same volume or weight, making them ideal for devices with high power requirements, such as smartphones, laptops, and electric vehicles.
Cycle Life
-
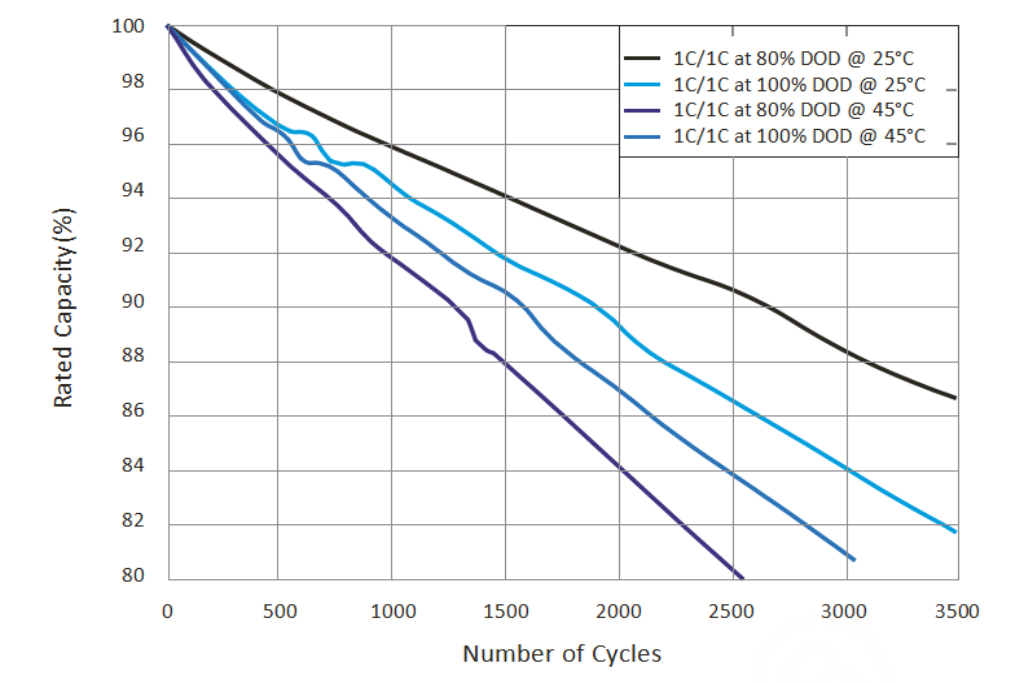
Regular Batteries: The cycle life of zinc-manganese dry batteries and other regular disposable batteries is virtually zero. Rechargeable nickel-cadmium batteries have a cycle life of about 500 - 1000 cycles. As the number of charge-discharge cycles increases, the electrode materials inside the battery undergo irreversible chemical changes, leading to a decrease in battery capacity.
-
Lithium Batteries: Lithium batteries have a relatively long cycle life. For instance, the cycle life of lithium iron phosphate batteries can exceed 2000 cycles, and some high-quality lithium batteries can last for 3000 - 5000 cycles under proper usage conditions. This means that lithium batteries can maintain good performance over a long period of use, reducing the frequency of battery replacement.
Self-Discharge Rate
-
Regular Batteries: The self-discharge rate of regular batteries is relatively high. For example, zinc-manganese dry batteries can lose 30% - 50% of their charge within a year of storage. This means that even if the battery is not in use, it will gradually lose its power.
-
Lithium Batteries: Lithium batteries have a lower self-discharge rate, with a monthly self-discharge rate of generally 5% - 10%. This means that lithium batteries can retain more of their charge after long periods of storage, making them more convenient to use.
III. Safety: Who is the More Reliable “Guardian”?
Regular Batteries
Regular batteries are relatively safe when used properly. However, if mishandled, for example, by leaving zinc-manganese dry batteries in a damp environment for an extended period, they may leak. The leaked electrolyte can corrode the surrounding devices and is also harmful to the human body.
Lithium Batteries
The safety of lithium batteries is more complex. The chemical substances inside lithium batteries are more reactive. If the battery is subjected to external impacts, short circuits, or overcharging, it may cause uncontrolled chemical reactions inside the battery, leading to fires or even explosions.
However, modern lithium batteries are usually equipped with various safety protection measures, such as overcharge protection, over-discharge protection, and short-circuit protection circuits, as well as safety valves inside the battery, to enhance battery safety.
IV. Application Fields: Each Shines in Its Own Way
Regular Batteries
Regular batteries are mainly used in small, low-power portable electronic devices, such as toys, small flashlights, and quartz clocks. These devices have low power requirements, and replacing batteries is relatively convenient.
Regular batteries are also widely used in one-time-use scenarios, for example, in temporary electronic device displays, where regular dry batteries can quickly provide power to the devices.
Lithium Batteries
The application range of lithium batteries is very extensive. In the field of consumer electronics, almost all smartphones, tablets, and laptops use lithium batteries as their power source. In the field of new energy vehicles, lithium batteries are the main power source for electric and hybrid vehicles.
In addition, lithium batteries are also used in some energy storage systems, such as solar power generation storage and wind power generation storage, to store excess electrical energy for use when needed.
V. Are Lithium Batteries Really More Durable?
From the perspective of cycle life and self-discharge rate, lithium batteries are clearly superior to regular batteries and are more suitable for applications that require multiple charge-discharge cycles and long-term storage.
However, in terms of usage environment, lithium batteries have higher requirements for temperature and humidity and need more safety protection measures. Regular batteries, although more tolerant of environmental conditions, have limited performance and lifespan.









 Network Supported
Network Supported