With the increasing demand for energy storage solutions, batteries have become the cornerstone of this technology. To help everyone gain a deeper understanding of this critical field, Lightboat Technology has compiled a comprehensive guide that breaks down the complexities of batteries into easy-to-understand concepts. By the end of this article, you'll have a solid grasp of battery technology and be well on your way to becoming an expert.
Enjoy your reading!I. The Battery Family
The term "lithium battery" originally referred to lithium metal batteries, which were single-use and prone to explosion, and thus have long been phased out.
Nowadays, what we commonly refer to as lithium batteries are actually lithium-ion batteries. The everyday 7号 and 5号 batteries we use are dry batteries; the old "brick" mobile phones, or "big brother" phones, used nickel-metal hydride batteries; small electric vehicles typically employ lead-acid batteries, which are often seen in groups of four, neatly packaged together; whereas our smartphones, laptops, and even electric vehicles mostly rely on lithium-ion batteries.
Comparison of characteristics of major electrochemical battery energy storage batteries.
II. Battery Terminology Explained
SOX: The full name is State Of X, which describes the state of the battery. H stands for Health, C for Capacity, P for Power, and E for Energy. It's somewhat similar to engine parameters like displacement, power, energy, and runtime. The general meaning is consistent.
SOC: (State of Charge) This refers to the charge level of the battery. Think of the battery's charge as water in a bucket. The amount of usable charge contained in the battery at a given moment is called the SOC at that moment. When the battery is completely discharged, the SOC is 0, and when it's fully charged, the SOC is 1. It's the usable capacity divided by the actual capacity.
DOD: (Depth of Discharge) This indicates the depth of discharge of the battery. When the battery is fully charged, its DOD is 0, and when it's completely discharged, the DOD is 1. So, under normal circumstances, the DOD of a battery is a value between 0 and 1, and the relationship between DOD and SOC is: DOD + SOC = 1.
SOH: (State of Health) This is the ratio of the battery's current actual capacity to its initial rated capacity. As the battery ages, the SOH will continuously decrease. It's generally measured based on capacity and internal resistance. The definition of SOH using battery capacity decay is the most common in literature, and it's given as follows:
Where: Caged is the current capacity of the battery; Crated is the rated capacity of the battery.
III. Classification of Lithium Batteries
A. By Performance
-
Power Type : Like a sprinter, it can output high power in a short time, suitable for devices that require instant high power output, such as some power tools.
-
Energy Type : Like a long-distance runner, it focuses on high energy storage, able to provide a stable supply of energy over a long period, commonly used in smartphones, laptops, and other devices that need extended battery life.
B. By Appearance
-
Cylindrical : With a regular shape and stable structure, and mature production processes. The 18650 lithium-ion battery, for example, is a cylindrical battery with a high degree of standardization, good safety, and is widely used in small electronic products and some electric vehicles.
-
Prismatic (Steel/Aluminum Case) : Can be customized in size according to actual needs, with relatively high space utilization, and is used in some devices where flexible space layout is required, such as in some laptop battery packs.
-
Pouch (Aluminum Plastic Film) : Light in weight and highly customizable in shape, it can be designed into various forms to fit the internal structure of different products. However, compared to steel or aluminum case batteries, its packaging strength is relatively lower. It is gradually being applied in some high-end electronic products with special requirements for battery shape and weight.
C. By Electrolyte Material
-
Liquid Lithium-ion Battery (LIB) : Uses liquid electrolyte and is currently widely used in power batteries due to its good conductivity and relatively high energy density. However, in terms of safety, certain protective measures are needed to prevent electrolyte leakage.
-
Polymer Lithium-ion Battery (PLB) : Replaces liquid electrolyte with solid polymer electrolyte, which can be in a "dry" or "gel" state. Compared to liquid lithium-ion batteries, polymer lithium-ion batteries have certain advantages in safety, as they are less likely to leak. They also have stronger shape adaptability and can better meet the needs of applications with special requirements for battery shape and safety. Solid-state batteries, strictly speaking, refer to those with both electrodes and electrolytes in solid form.
D. By Cathode Material
-
Lithium Iron Phosphate Battery (LFP) : It has a moderate working voltage (3.2V), large electrical capacity (170mAh/g), high discharge power, fast charging capability, long cycle life, and high stability in high-temperature and high-heat environments. Moreover, it doesn't contain expensive elements like cobalt. The raw materials are low in cost and abundant in resources, making it widely used in energy storage fields and some power batteries with high safety requirements.
-
Lithium Cobalt Oxide Battery (LCO) : It has a higher energy density and can provide higher voltage and better battery life. However, the cost is relatively high, and its safety is relatively weaker compared to other types. It is prone to overheating issues. It was widely used in early mobile phone batteries and other consumer electronics. But with the development of technology and increasing safety requirements, its application scope has been somewhat limited.
-
Lithium Manganese Oxide Battery (LMO) : It has certain cost advantages and better low-temperature performance. However, its energy density is relatively low, and its cycle life is slightly inferior to that of lithium iron phosphate batteries. It is used in some small power tools and low-end electric vehicles.
-
Bipolar Battery : Including nickel-manganese cobalt oxide and nickel-cobalt oxide, etc. By combining different metal elements, it can balance performance indicators such as energy density and safety to a certain extent. However, its market share is relatively small at present.
-
Ternary Battery : Including nickel-cobalt-manganese cobalt oxide (NCM) and nickel-cobalt-aluminum cobalt oxide (NCA). They have high energy density and can meet the requirements of electric vehicles and other applications with high demands for driving range. But in terms of safety, more precise management and control are needed. They are widely used in mid-to-high-end electric vehicles and some consumer electronics.
E. By Anode Material
-
Lithium Titanate Battery (LTO) : It has excellent cycle life and safety, and can maintain better performance under fast charging and discharging conditions. However, its energy density is relatively low. It is mainly used in some special fields where battery life and safety are required to be extremely high, but the energy density requirements are relatively low, such as some energy storage power stations.
-
Graphene Battery : Graphene has a unique two-dimensional structure and excellent electrical conductivity. Using it as the anode material of batteries is expected to significantly improve the charging and discharging speed and energy density of batteries. However, it is currently still in the research and gradual promotion stage, facing challenges such as cost control and process optimization.
-
Nano Carbon Fiber Battery : Nano carbon fibers have a large specific surface area and good electrical conductivity, which can provide more active sites for the embedding and desorption of lithium ions, thereby improving the performance of the battery. It is also being researched and explored, and is expected to be applied in some high-performance battery fields in the future.
IV. Lithium Battery Voltage and Capacity
The voltage of lithium-ion batteries varies with discharge current, ambient temperature, and different cathode and anode materials.
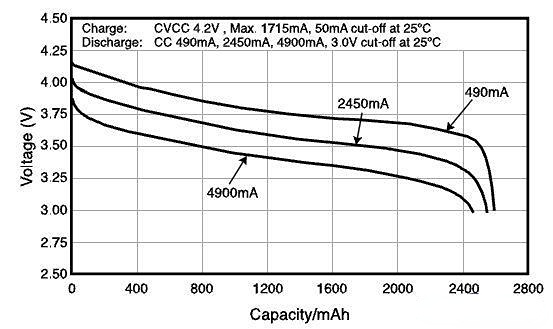
This chart shows the discharge curve of a Panasonic 2550mAh lithium-ion battery using lithium cobalt oxide as the cathode material (insert the discharge curve chart here). The three curves from top to bottom represent the changes in voltage and capacity when using three different discharge currents. First of all, the voltage changes continuously during the charging and discharging process. Taking 490mA as an example, the open-circuit voltage of the fully charged battery is 4.2V. As discharge progresses, the voltage (vertical axis) decreases slowly, and the discharged electricity (horizontal axis) increases gradually, until the voltage starts to drop sharply at 3.5V. Although the voltage changes throughout the discharge process, for simplicity, only the average value of the relatively flat discharge part of the curve, 3.7V, is labeled as the battery voltage. This section of voltage is also called the nominal voltage. This voltage is measured under conditions of low current and room temperature, and it will decrease with increasing discharge current and decreasing temperature. Another important factor that affects battery voltage is the cathode and anode materials. The Panasonic battery mentioned above uses lithium cobalt oxide and graphite as the cathode and anode materials respectively, which was the standard material for the entire lithium battery industry a few years ago. With the application of new materials in batteries, some 3.6V or 3.8V lithium batteries have emerged in recent years. They use different cathode materials. Compared with lithium cobalt oxide batteries, they can increase energy density, that is, store more electricity in a unit of weight and volume.
| Battery Type |
Charge Cut-off Voltage |
Nominal Voltage |
Discharge Cut-off Voltage |
| Lithium-ion Battery |
4.2V |
3.7V |
2.7V |
| LiFePO₄ (LFP) Battery |
3.6V |
3.2V |
2.0V |
Battery capacity is divided into rated capacity and actual capacity.
-
The actual capacity refers to the actual amount of electricity discharged by the battery under certain discharge conditions. The actual capacity is always lower than the theoretical capacity.
-
The rated capacity refers to the minimum amount of electricity that the battery should discharge under certain discharge conditions as specified in the design and manufacturing of the battery. Battery capacity is generally calculated in AH (ampere-hours, ampere-hours). For the sake of convenience, single-cell batteries are usually labeled in mAh (milliampere-hours). If the rated capacity of a battery is 1300mAh, that is, if the battery is discharged with a current of 130mA, then the battery can work continuously for 10 hours (1300mAh/130mA = 10h). This is an analysis under ideal conditions. The current in digital devices cannot always be kept constant at a certain value during actual operation. The capacity of 18650 lithium batteries generally ranges from 1200mAh to 3600mAh. The unit for measuring mobile phone battery capacity now is mAh. High school knowledge tells us that this is a unit of electric charge, and the voltage needs to be multiplied to get the unit of energy.
The method for calculating battery capacity :
The method for calculating battery energy :
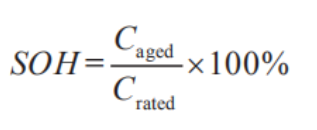

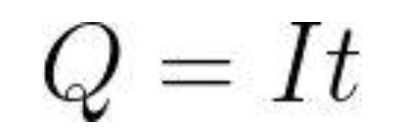
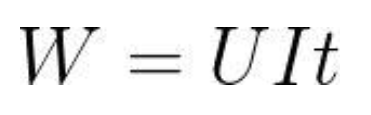
 Network Supported
Network Supported